Chemical Bonding: Definition, Types, Properties
Content:
Chemical bonding, its types, properties, along with chemical reactions is one of the cornerstones of an interesting science called chemistry. In this article, we will describe all aspects of chemical bonds, their importance in science and much more.
Definition
What is a chemical bond? The chemical bond is the mutual adhesion of atoms in a molecule and the crystal lattice, as a result of the action of the force of attraction that exists between atoms. It is thanks to chemical bonds that various chemical compounds are formed; this is the nature of the chemical bond.
Formation
The basic answer is that atoms are trying to reach the most stable (lowest-energy) state that they can. Many atoms become stable when their valence shell is filled with electrons or when they satisfy the octet rule (by having eight valence electrons). If atoms don’t have this arrangement, they’ll “want” to reach it by gaining, losing, or sharing electrons via bonds. So now you know the answer to the question “what causes a chemical bond to form between atoms?”
Types
The mechanism of the formation of a chemical bond strongly depends on its type. In general, there are 4 types of chemical bonds:
- Covalent chemical bond (which in turn can be polar and non-polar)
- Ionic bond
- Hydrogen bond
- Metallic bond
As for the covalent chemical bond, there is a separate article on our website, and you can read more in the link. We only notice that the covalent bond is the strongest chemical bond. Further, we will describe in more detail all the other main types of chemical bonds.
Ionic Bond
Ionic bonds form when the mutual electric attractions of two ions have different charges. In such chemical bonds ions usually are simple, consisting of one atom of the substance.
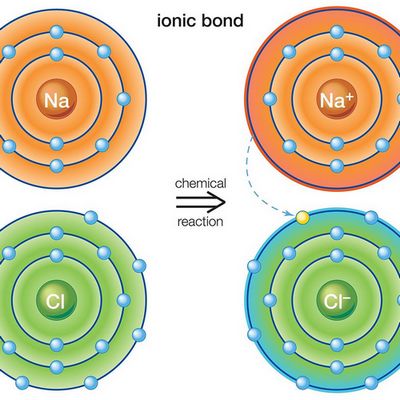
Characteristics properties of ionic bonds are the absence of saturation in it. As a result, a very different amount of oppositely charged ions can join an ion or even a whole group of ions. The ionic bond example is cesium fluoride compound CsF, in which the level of “ionity” is almost 97%.
Hydrogen Bond
Long before the modern theory of chemical bonds was form scientists-chemists noticed that hydrogen compounds with non-metals possess various surprising properties. For example, the boiling temperature of the water with hydrogen fluoride is much higher than it could be. This is a good example of a hydrogen bond.
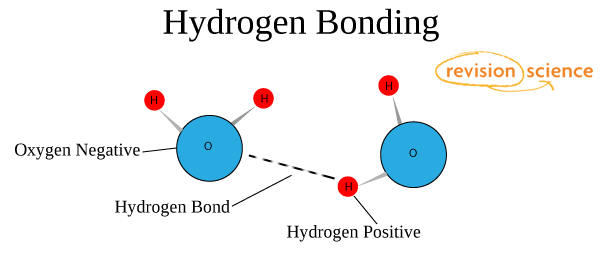
This picture shows the formation of a hydrogen chemical bond.
The nature and properties of the hydrogen bond are due to the ability of the hydrogen atom H to form another chemical bond. The reason for the formation of such a bond is the properties of electrostatic forces. For example, the total electron cloud in a molecule of hydrogen fluoride is so biased towards fluorine that the space around the atom of this substance is saturated with a negative electric field. Around the hydrogen atom, deprived of its only electron, everything is exactly the opposite, and its electron field is much weaker and, as a result, has a positive charge. Positive and negative charges are attracted, so in such a simple way a hydrogen bond forms.
Metallic Bond
Metals have their own type of chemical bond – the atoms of all metals are located in a certain way. The order of their arrangement is called a crystal lattice. The electrons of different atoms form a common electron cloud, while they weakly interact with each other.
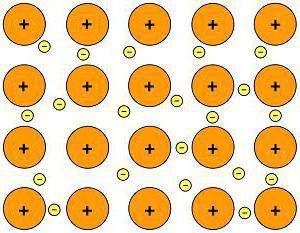
This is a metallic bond.
If you see a broken fence it could mean that metallic bonds are broken. Yet this is not the problem because you can order metal fence repair at quickfencerepair.com.
How to Determine the Type of Bond?
The type of chemical bond depends on the substances taking part in it. If the substances are metal and non-metal, then the bond between them is ionic. If two substances are metal, then the bond between them is metallic. If two substances are non-metal, then the bond is covalent.
Properties
Different quantitative characteristics are used to compare different chemical reactions such as:
- length
- energy,
- polarity
- the order.
Let us describe them in more detail.
The bond length is the equilibrium distance between the nuclei of atoms that are connected by a chemical bond.
The energy of a chemical bond determines its strength. In this case, the energy means the effort required to break the chemical bond and to separate the atoms.
The polarity of a chemical bond shows how much the electron density is shifted to one of the atoms. The ability of atoms to shift their electron density towards themselves or, in simple terms, “pull a blanket over themselves” is called electronegativity in chemistry.
The order of a chemical bond (in other words, the multiplicity of a chemical bond) is the number of electron pairs entering into a chemical bond. The order can be both integer and fractional, the higher it is, the greater the number of electrons carries out chemical bonding and the more difficult it is to break it.
References and Further Reading
- Rioux, F. (2001). “The Covalent Bond in H2”. The Chemical Educator. 6 (5): 288–290. doi:10.1007/s00897010509a.
- Lewis, Gilbert N. (1916). “The Atom and the Molecule”. Journal of the American Chemical Society. 38 (4): 772. doi:10.1021/ja02261a002. a copy
- Бор Н. (1970). Избранные научные труды (статьи 1909–1925). 1. М.: «Наука». p. 133.
- Svidzinsky, Anatoly A.; Marlan O. Scully; Dudley R. Herschbach (2005). “Bohr’s 1913 molecular model revisited”. Proceedings of the National Academy of Sciences. 102 (34[1]): 11985–11988. arXiv:physics/0508161. Bibcode:2005PNAS..10211985S. doi:10.1073/pnas.0505778102. PMC 1186029. PMID 16103360.
- Laidler, K. J. (1993). The World of Physical Chemistry. Oxford University Press. p. 346. ISBN 978-0-19-855919-1.
- James, H.H.; Coolidge, A S. (1933). “The Ground State of the Hydrogen Molecule”. Journal of Chemical Physics. 1 (12): 825–835. Bibcode:1933JChPh…1..825J. doi:10.1063/1.1749252.
- “Bond Energies”. Chemistry Libre Texts. Retrieved 2019-02-25.
- Atkins, Peter; Loretta Jones (1997). Chemistry: Molecules, Matter and Change. New York: W.H. Freeman & Co. pp. 294–295. ISBN 978-0-7167-3107-8.

Author: Pavlo Chaika, Editor-in-Chief of the journal Poznavayka
When writing this article, I tried to make it as interesting and useful as possible. I would be grateful for any feedback and constructive criticism in the form of comments to the article. You can also write your wish/question/suggestion to my mail pavelchaika1983@gmail.com or to Facebook.


This good to know about chemical bonding.