Chemosynthesis
Content:
Definition
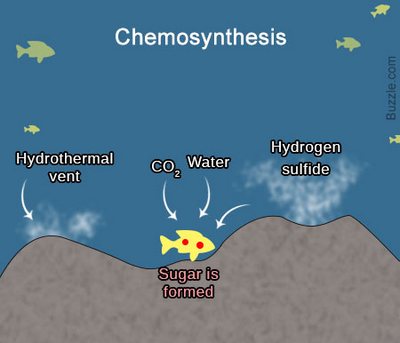
What is chemosynthesis? The process of chemosynthesis is a unique phenomenon in biology. Chemosynthesis is an unusual type of nutrition of bacteria, based on the assimilation of carbon dioxide СО2 due to the oxidation of inorganic compounds. According to scientists, chemosynthesis is the oldest type of autotrophic nutrition (such nutrition, when the body itself synthesizes organic matter from inorganic), which could appear even earlier than photosynthesis.
Discovery
As a biological phenomenon, chemosynthesis of bacteria was discovered by the Russian biologist S. Vinogradsky in 1888. The scientist has proven the ability of some bacteria to release carbohydrates using chemical energy. He also singled out a number of specific chemosynthetic bacteria; sulfur bacteria, iron bacteria and nitrifying bacteria are the most prominent among them.
Similarities and Differences With Photosynthesiss
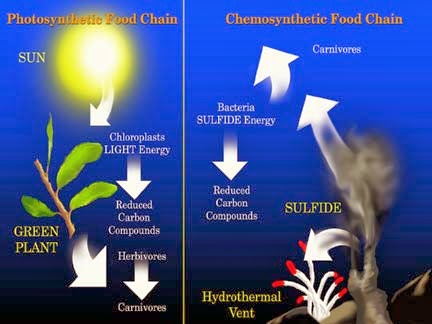
Let us now analyze the similarities of photosynthesis and chemosynthesis, and what is the differences between them.
Similarity:
- Both chemosynthesis and photosynthesis are types of autotrophic nutrition, when the body releases organic matter from inorganic.
- The energy of such a reaction is stored in adenosine triphosphoric acid (abbreviated ATP) and subsequently used for the synthesis of organic substances.
The difference between photosynthesis and chemosynthesis:
- They have a different source of energy, and as a result, different redox reactions. During chemosynthesis, the primary source of energy is not sunlight, but a chemical reaction of oxidation of certain substances.
- During chemosynthesis, the bacterial cells don’t have the chlorophyll (the green pigment); during photosynthesis, on the contrary, they have the chlorophyll.
- In chemosynthesis, the source of carbon for the synthesis of organics can be not only carbon dioxide but also carbon monoxide (CO), formic acid, acetic acid, methanol, and carbonates.
What is the Source of Energy?
Where do chemosynthetic bacteria get their energy? Chemosynthetic bacteria get their energy due to the oxidation of hydrogen, manganese, iron, sulfur, ammonia, etc. Depending on the substrate being oxidized, the bacteria got their names: iron bacteria, sulfur bacteria, methane-forming archaea, nitrifying bacteria, and so on.
Where Does Chemosynthesis Occur?
Chemotrophs is the organisms that receive vital energy through chemosynthesis. Chemotrophs play an important role in the circulation of substances, especially nitrogen. In particular, they support soil fertility. Besides this, large reserves of ore and nitrate are accumulated under natural conditions due to the activity of chemosynthetic bacteria.
Examples
In addition to bacterial and archaea, some larger organisms rely on chemosynthesis. A good example is the giant tube worm which is found in great numbers surrounding deep hydrothermal vents. Each worm houses chemosynthetic bacteria in an organ called a trophosome. The bacteria oxidize sulfur from the worm’s environment to produce the nourishment the animal needs.

This is the photo of the giant tube worm.
Equation
Now let’s take a closer look at the existing chemosynthesis reactions and their equations; they all differ depending on the chemosynthetic bacteria.
Iron Bacteria
Iron bacteria include filamentous and iron-oxidative leptotriks, spherotillus, gallionella, metallogenium. They live in fresh and marine waters. Iron bacteria form iron ore deposits due to the chemosynthesis reaction of oxidation of ferrous iron to ferric.
4FeCO3 + O2 + 6H2O → Fe(OH)3 + 4CO2 + E (energy)
In addition to energy, carbon dioxide is produced in this reaction.
Sulfur Bacteria
The other name of sulfur bacteria is thiobacteria; it is a very large group of microorganisms. These bacteria gain energy by oxidizing compounds with reduced sulfur.
2S + 3O2 + 2H2O → 2H2SO4 + E
The sulfur may accumulate in the bacteria, or be released into the environment in the form of flakes.
Nitrifying Bacteria
These bacteria (which live in the earth and water) receive their energy through ammonia and nitrous acid; they play a very important role in the nitrogen circulation.
2NH3 + 3O2 → HNO2 + 2H2O + E
Nitrous acid, obtained by this reaction, forms salts and nitrates in the earth, contributing to its fertility.
References and Further Reading
- Julian Chela-Flores (2000): “Terrestrial microbes as candidates for survival on Mars and Europa”, in: Seckbach, Joseph (ed.) Journey to Diverse Microbial Worlds: Adaptation to Exotic Environments, Springer, pp. 387–398. ISBN 0-7923-6020-6
- Biotechnology for Environmental Management and Resource Recovery. Springer. 2013. p. 179. ISBN 9788132208761.
- Campbell N.A. e.a. (2008) Biology 8. ed. Pearson International Edition, San Francisco. ISBN 978-0-321-53616-7
- The purple phototrophic bacteria. Hunter, C. Neil, 1954-. Dordrecht: Springer. 2009. ISBN 9781402088148. OCLC 304494953.
- Kellerman, M.Y., G. Wegener, M. Elvert, M.Y. Yoshinaga, Y-S. Lin, T. Holler, X.P. Millar, K. Knittel, and K-U. Hinrichs (2012). Autotrophy as a predominant mode of carbon fixation in anaerobic methane-oxidizing microbial communities. Proc. Natl. Acad. Sci. USA 109(47):19321-19326

Author: Pavlo Chaika, Editor-in-Chief of the journal Poznavayka
When writing this article, I tried to make it as interesting and useful as possible. I would be grateful for any feedback and constructive criticism in the form of comments to the article. You can also write your wish/question/suggestion to my mail pavelchaika1983@gmail.com or to Facebook.

